Abstract
Introduction: In the BABY HUG trial, fixed-dose hydroxyurea (20 mg/kg/day) was safe and effective in decreasing sickle cell anemia (SCA)-related complications in very young children (9-18 months). Nevertheless, children in the treatment arm of BABY HUG continued to experience vaso-occlusive symptoms and incur organ damage. In older children/adults with SCA, intensification of hydroxyurea to a maximum tolerated dosage, defined by mild to moderate myelosuppression, has been associated with improved laboratory parameters and clinical benefits compared to fixed lower dosing. However, relatively little has been published regarding hydroxyurea dose escalation in infants with SCA. We designed the Hydroxyurea Management in Kids: Intensive versus Stable Dosage Strategies (HUGKISS) to determine the feasibility of enrolling, randomizing, and treating very young children with SCA with either a fixed or intensified dose of hydroxyurea.
Methods: We performed a single-blind, randomized, multicenter pilot trial of hydroxyurea in infants and toddlers (9-36 months old) with SCA to compare a "standard" treatment (20 mg/kg/day) with an "intensive" treatment defined by a goal absolute neutrophil count (ANC) of 1500-3000 cells/µL. Participants were randomized in a 1:1 ratio to receive "standard" or "intensive" therapy based on a block randomization and were stratified by clinical center and age (9 to <24 months or 24 to <36 months). In both the standard and intensive treatment arms, participants began hydroxyurea at 20 (±2.5) mg/kg/day. There was no dose escalation in the HUGKISS standard arm. Participants treated in the intensive arm were dose-escalated by 5 mg/kg/day increments (if tolerated) to a maximum of 35 mg/kg/day, adjusted every 8 weeks to maintain the goal ANC. Participants underwent clinical, laboratory, and medication adherence assessments every 4 (±2) weeks. The medical coordinating center staff and study center principal investigators were blinded to treatment allocation, while hydroxyurea volume was not blinded to participants or caregivers.
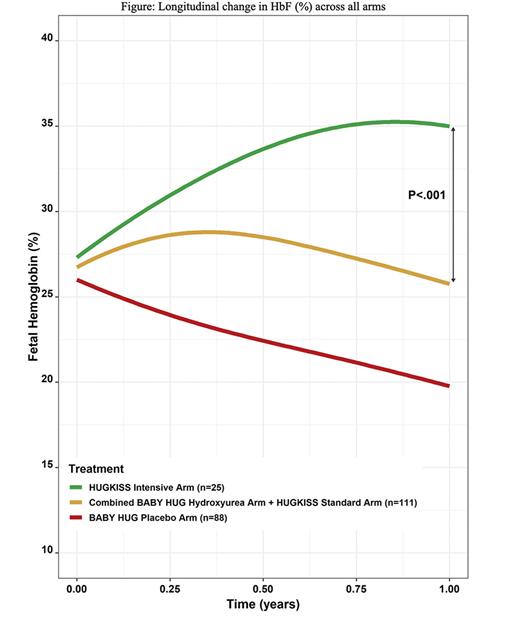
Results: Twenty-six subjects were randomized to standard treatment and 25 to intensive treatment, with no statistical difference in the subjects' mean age at enrollment (12.8 months) or laboratory values at randomization. One year after randomization, 19 and 23 subjects were evaluable in the standard and intensive arms respectively. The mean hydroxyurea doses at exit were 27.2 mg/kg/day (intensive arm) and 18.8 mg/kg/day (standard arm). Significantly greater increases in fetal hemoglobin (HbF) were noted in the intensive versus standard arm (p=0.002, final levels, 38.8% vs 26.1%), and the median hemoglobin (Hb) (p=0.033, +1.2g/dl vs +0.4 g/dl). Significantly greater increases in mean corpuscular volume and greater decreases in reticulocyte count and ANC also occurred in the intensive arm. Significant differences were not seen in SCA-related adverse event rates. The intensive arm had more frequent neutropenic toxicity (ANC<1000 cells/µL) than the standard arm (52% versus 23%), but no serious infections occurred. Data from the BABY HUG treatment arm were pooled with our standard dose arm since both groups received the same dose of hydroxyurea and were of similar age. When the data were summarized and compared in longitudinal curves (Figure), similar differences from our intensive arm were found, often within 6 months of dose initiation.
Conclusions: Intensive hydroxyurea treatment induced greater increases in HbF and Hb than standard dosing without significant toxicity in very young children with SCA, and may blunt or delay the fall in HbF seen after 6 months of therapy with lower doses. HUGKISS demonstrated the feasibility of treating very young children with SCA with intensive dose hydroxyurea.
Disclosures
Brown:Novartis: Consultancy, Research Funding; Pfizer: Research Funding; Imara: Consultancy, Research Funding; Forma Therapeutics: Research Funding; Novo Nordisk: Consultancy; Global Blood Therapeutics: Consultancy, Current Employment, Current equity holder in publicly-traded company, Research Funding. Rogers:Magenta Therapeutics: Consultancy; Novartis: Consultancy. Hankins:CVS Health: Consultancy; Forma Therapeutics: Consultancy; GBT: Consultancy; NHLBI: Membership on an entity's Board of Directors or advisory committees, Research Funding; CDC: Research Funding; HRSA: Research Funding; ASH: Membership on an entity's Board of Directors or advisory committees. Porter:Forma Therapeutics: Consultancy. Estepp:Eli Lilly and Co: Research Funding; Pfizer: Research Funding; Global Blood Therapeutics: Consultancy, Research Funding; Emmaus Life Sciences: Consultancy; Daiichi Sankyo: Consultancy; Agios: Current Employment, Current equity holder in private company; Forma Therapeutics: Research Funding.
OffLabel Disclosure:
Hydroxyurea for the management of sickle cell disease in very young children
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal